Rare earth metals are actually not as rare as their name might imply. They are critical to high-performance optics and lasers, and essential to the most powerful magnets and superconductors in the world.
Rare earths are simply more expensive to mine than most metals when not mined with environmentally harmful chemicals. These metals are also traditionally not as profitable in the markets. This has made them less desirable in the past—until the world realized that China controlled much of the market.

These difficulties, combined with the demand for the metals for use in high-tech applications, introduces economic and political complications that make some of the most interesting metals even more exciting for investors.
Rare Earths in the Marketplace
According to the United States Geological Survey, as of 2018, China produced around 80% of world demand for rare earth metals (down from 95% in 2010). Their ores are rich in yttrium, la, and neodymium.
Since August of 2010, fears over Chinese dominance of crucial rare earth supplies have lingered as China restricted export quotas of the metals with no official explanation, immediately sparking debate over decentralization of world rare earth production.
Great quantities of rare earth ores were found in California in 1949, and more are being sought throughout North America, but current mining is not significant enough to strategically control any portion of the global rare earths market (the Mountain Pass mine in California still has to ship its minerals to China to be processed).
Rare earths are traded on the NYSE in the form of exchange-traded funds (ETFs) that represent a basket of supplier and mining stocks, as opposed to trading in the metals themselves. This is due to their rarity and price, as well as their almost strictly industrial consumption. Rare earth metals are not considered a good physical investment like precious metals, which hold low-tech intrinsic value.
Rare Earth Metals and Their Applications
In the periodic table of the elements, the third column lists the rare earth elements. The third row of the third column is expanded below the chart, listing the lanthanide series of elements. Scandium and Yttrium are listed as rare earth metals, although they are not part of the lanthanide series. This is due to the prevalence of the two elements being similar in part to the lanthanides.
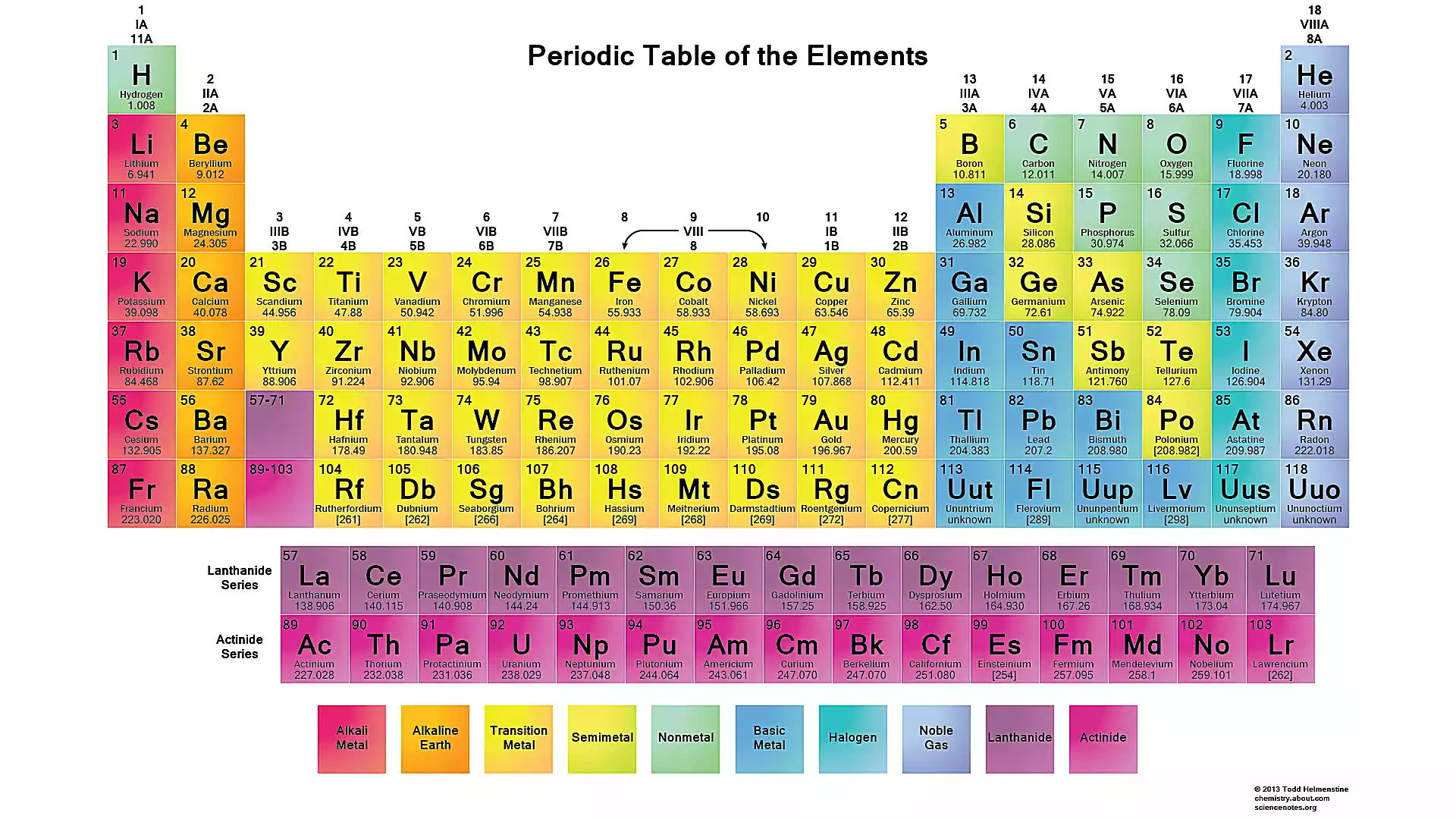
Column 3 of the Periodic Table of Elements Lists the Rare Earths. Todd Helmenstine
In order of increasing atomic mass, the 17 rare earth metals and some of their common applications are given below.
Copyright © 2020. we All rights reserved.